










Jinarc® is a prescription drug registered to Hong Kong Department of Health. Professional advice from doctors should be strictly followed when taking these drugs. For enquiry, please consult your doctor or pharmacist.


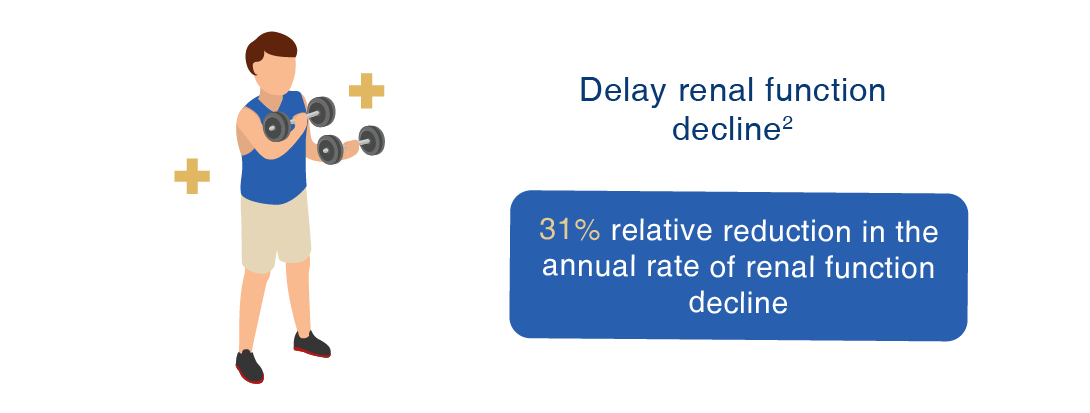
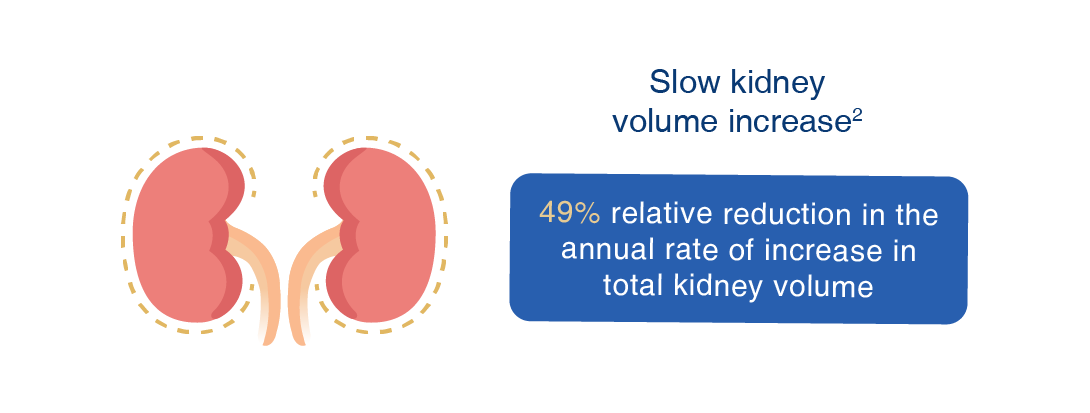
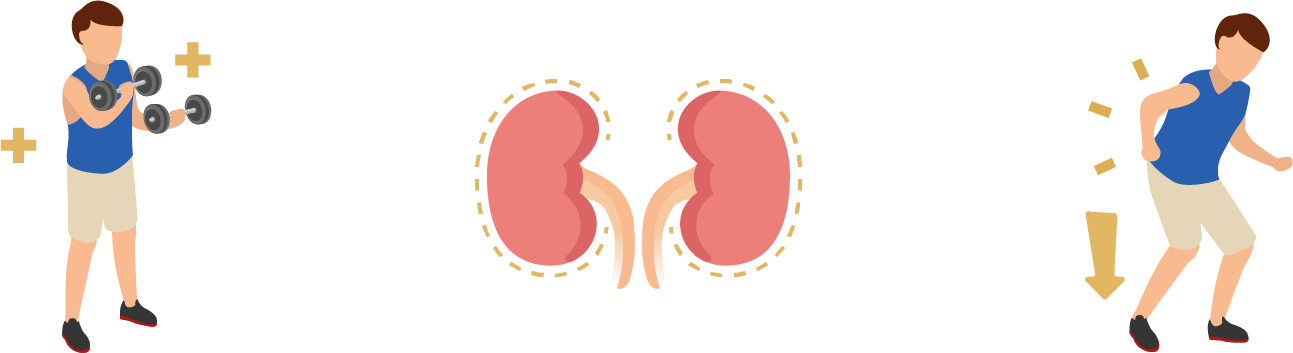
Jinarc® has been proven effective in clinical studies to:


Jinarc® is a prescription drug registered to Hong Kong Department of Health. Professional advice from doctors should be strictly followed when taking these drugs. For enquiry, please consult your doctor or pharmacist.





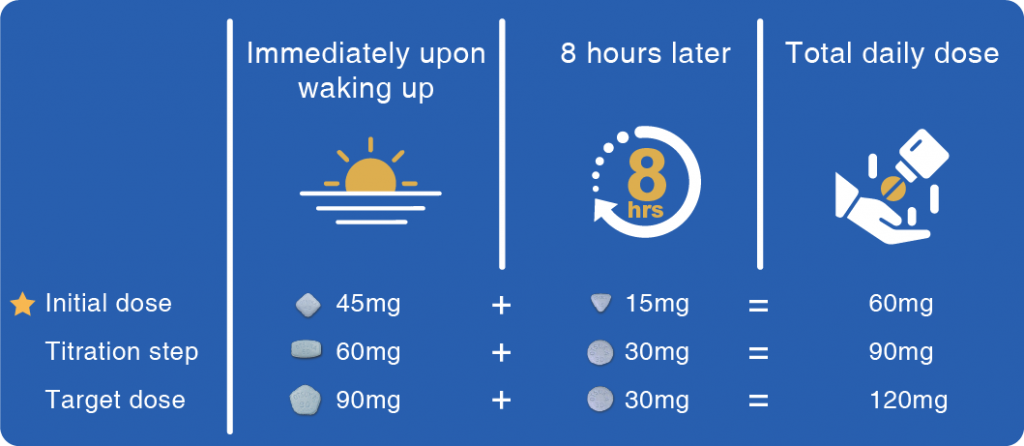


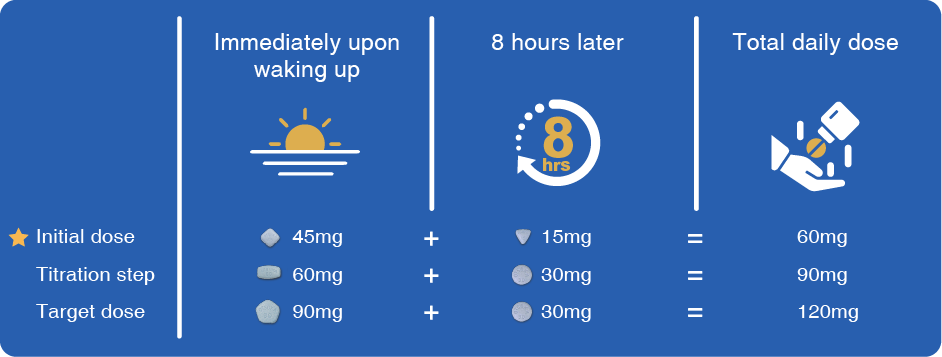
If you are prescribed Jinarc®, you can refer to the below dosing information:




























A: Jinarc® may cause adverse reactions related to water loss such as thirst, polyuria, nocturia, and pollakiuria. Hence, patients have to be instructed to drink water or other aqueous fluids at the first sign of thirst in order to avoid excessive thirst or dehydration. Additionally, patients have to drink 1-2 glasses of fluid before bedtime regardless of perceived thirst and replenish fluids overnight with each episode of nocturia.
A: Please consult a doctor before taking other medications. From clinical data, Jinarc® has been known or suspected for interactions with drugs categorized as moderate or strong CYP3A inhibitors.
*Examples of moderate CYP3A inhibitors include amprenavir, aprepitant, atazanavir, ciprofloxacin, crizotinib, darunavir/ritonavir, diltiazem, erythromycin, fluconazole, fosamprenavir, imatinib, verapamil; examples of strong CYP3A inhibitors include itraconazole, ketoconazole, ritonavir, clarithromycin.
A: Jinarc® is generally well tolerated: thirst, polyuria, nocturia, and pollakiuria may occur. Jinarc® has minor influence on the ability to drive or use machines. However, when driving vehicles or using machines it has to be taken into account that occasionally dizziness, asthenia or fatigue may occur.
A: People who have had an allergic reaction to the active substance tolvaptan, any of the excipients of Jinarc® , benzazepine or benzazepine derivatives should not take it. Also, patients with anuria, volume depletion, hypernatraemia, patients who cannot perceive or respond to thirst, and patients who are pregnant or breast-feeding should not take it. Moreover, patients with elevated liver enzymes and/or signs or symptoms of liver injury prior to initiation of treatment that meet the requirements for permanent discontinuation of Jinarc® should not take it as well. For enquiry, please consult your doctor or pharmacist.

A: Jinarc® may cause adverse reactions related to water loss such as thirst, polyuria, nocturia, and pollakiuria. Hence, patients have to be instructed to drink water or other aqueous fluids at the first sign of thirst in order to avoid excessive thirst or dehydration. Additionally, patients have to drink 1-2 glasses of fluid before bedtime regardless of perceived thirst and replenish fluids overnight with each episode of nocturia.
A: Most importantly, you must not drink grapefruit juice during the course of Jinarc® treatment.
On the other hand, there is limited clinical data on the impact of alcohol and smoking on the efficacy and safety of Jinarc®. Nevertheless, we recommend to adopt a healthy lifestyle which is good to your conditions.
A: Please consult a doctor before taking other medications. From clinical data, Jinarc® has been known or suspected for interactions with drugs categorized as moderate or strong CYP3A inhibitors.
*Examples of moderate CYP3A inhibitors include amprenavir, aprepitant, atazanavir, ciprofloxacin, crizotinib, darunavir/ritonavir, diltiazem, erythromycin, fluconazole, fosamprenavir, imatinib, verapamil; examples of strong CYP3A inhibitors include itraconazole, ketoconazole, ritonavir, clarithromycin.
A: Jinarc® is generally well tolerated: thirst, polyuria, nocturia, and pollakiuria may occur. Jinarc® has minor influence on the ability to drive or use machines. However, when driving vehicles or using machines it has to be taken into account that occasionally dizziness, asthenia or fatigue may occur.
A: People who have had an allergic reaction to the active substance tolvaptan, any of the excipients of Jinarc® , benzazepine or benzazepine derivatives should not take it. Also, patients with anuria, volume depletion, hypernatraemia, patients who cannot perceive or respond to thirst, and patients who are pregnant or breast-feeding should not take it. Moreover, patients with elevated liver enzymes and/or signs or symptoms of liver injury prior to initiation of treatment that meet the requirements for permanent discontinuation of Jinarc® should not take it as well. For enquiry, please consult your doctor or pharmacist.
References :
1. Jinarc Hong Kong prescribing information. May 2020.
2. Torres VE, et al. N Engl J Med. 2012 Dec 20;367(25):2407-18.
3. Jinarc Patient Education Brochure. Aug 2020.
4. PKD Charity: Diet and Lifestyle. Available at: https://www.pkdcharity.org.uk/about-adpkd/living-with-adpkd/diet-and-lifestyle. Accessed: 20 Apr 2021.
Jinarc® is a registered trademark of Otsuka Pharmaceutical Co., Ltd. of Japan.
The information on this webpage only includes general information, which is only for the personal reference of those who visit this website, and does not replace the professional advice and instructions given to patients by doctors or pharmacists. For details, please consult your doctor or pharmacist. You should not rely solely on the content contained in this webpage, and we assume no responsibility or consequences for any omissions or errors in the content. Otsuka Pharmaceutical (H.K.) Limited does not endorse or recommend any products disclosed or displayed on this website, and does not make any representations or guarantees regarding any information contained. The information on this website is only applicable to people who visit this website in Hong Kong, and the information contained about the drug may be different from the drug labels of other countries.
Medical professionals should refer to the detailed prescribing information of the product registered with the Pharmacy and Poisons Board of Hong Kong for complete product information. The information published on this website is not a substitute for your medical judgment. We kindly ask medical staff to evaluate the information provided on this website with professional judgment before making any evaluation or treatment decisions. Under any circumstances, Otsuka Pharmaceutical (H.K.) Limited shall not be liable for any damage, loss or injury caused by using or relying on the information provided on this website in any form.
HKOP-JIN-202109-001










